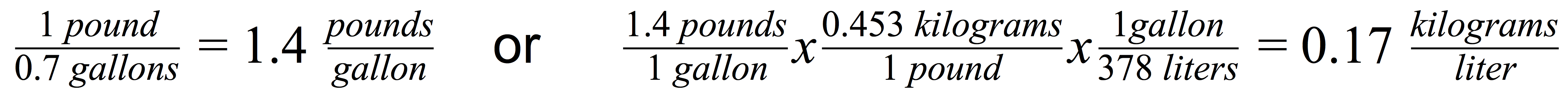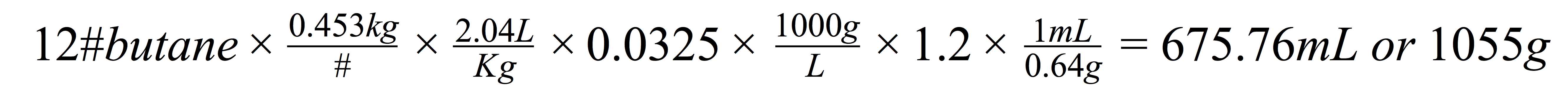“Simplicity, simplicity, simplicity” said Henry David Thoreau…
In any process, it’s best to simplify it if you can. In any math class, you want to simplify your algebra before doing more complex math. This is a matter of time, efficiency, and of course money. What I’m proposing here is that people simplify and perhaps improve their extraction process.
No doubt, hydrocarbon extractions are a risky business. On the small scale, you anywhere up to 12 pounds of butane in-line to your extractor at any given time. That has a significant explosion potential – the endless news stories of people blowing up houses describe it well enough.
Since most extractors have dry ice on hand to freeze their plant material or to control temperatures of their butane, you can just as well use some of that dry ice to concentrate your trichomes. This may seem like more work on the front end, but it does save you work in the long run, and is also much safer since it means less time cycling butane through your extractor.
It’s a matter of concentration (i.e. density).
In my former life as a protein chemist, concentration was everything. Concentration is described by the number of Moles of any substance, divided by the the volume (or the amount of liquid) the substance is dissolved in. Concentration is a close cousin to density. For the sake of our discussion, we’ll talk in terms of density.
Density can be described as the amount of weight divided by the amount of space it takes up (i.e. volume) – e.g. pounds/gallon or kilograms/liter. Now imagine that you have 1 pound of buds, and that takes up a space of a 3”x36” column. A 3”x36” column has a volume approximately 0.7 gallons. This works out to be 1.4 pounds per gallon or 0.17 kilograms per liter.
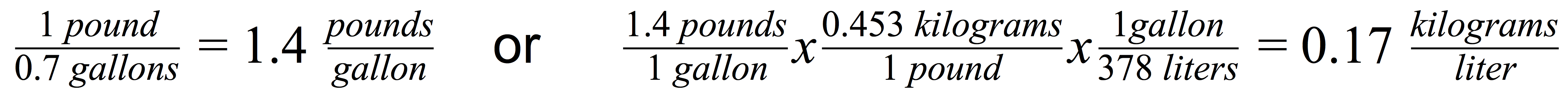
Now take that same volume of buds and make dry ice hash with it. Your yields will vary depending on the strain, but you can expect anywhere from 15-25% yields. This will produce a much smaller volume of concentrated trichomes. You don’t have to do much math to understand that weight for weight, a column packed with trichomes has more cannabinoids and terpenes compared to a column packed with nugs or trim.
To further the point, the density of cannabinoids and terpenes is much higher in a pile of trichomes than is in a equally sized pile of nugs. The point is that by concentrating – ie increasing the cannabinoid and terpene content while decreasing the volume – you increase the efficiency of your extraction.
Your goal is to get the highest yield dry ice extraction as possible.
In this case, you don’t need to make perfect, connoisseur grade, bubble hash. The amount of plant matter picked up is insignificant to the process since it will be filtered out during extraction.
So you’ve just taken 1 pound of dried buds that takes up 0.7 gallons of space and turned it into what looks like a powder that weighs 0.15-0.25 pounds and takes up something like 0.1 gallons of space. This defines the verb “to concentrate” as known by a chemist – you’ve just simplified your algebra. Now you have a product that will likely fit into a 3”x6” column, and can be run through the extractor.
The great part about this method is that you can filter out the plant material with your filter plates. You end up with a fine oil that is rich in terpenes and cannabinoids while limiting the amount of waxes and other phytochemicals that would be extracted from plant material. This is a major increase in efficiency.
Materials and preparations for making dry ice hash.
Dry ice hash is relatively easy to make. The tools of the trade are a 220 micron bubble bag, dry ice, and a flat surface to shake it out onto. A simple way of doing this is setting up a 2 bucket system, where one bucket fits into another. The top bucket will have its bottom cut out, and the 220 micron bag slips over it. The second bucket, with it’s bottom intact, fits over the 220 micron bag that’s wrapped over the cut out bucket, and is where you collect the trichomes. Pull the bag tight over the cutout and tie off the string on the bag to keep it tight against the bucket.
Dry ice costs $1.25/pound from the local grocery store, so the material cost is relatively inexpensive. You can be generous with the dry ice. A 1:1 ratio of dry ice to buds is the higher end, and you can get by with less, but you may sacrifice yields. Freeze your buds for 48 hours in the freezer before hand. Alternatively, you can just as well let the dry ice freeze them.
Making dry ice hash.
Break up your dry ice into 1” chunks to increase the surface area coming into contact with the plant material. Place the 220 micron bag over the cut out bucket and then put your buds into the 220 micron bag along with the dry ice. Gently stir the buds around with the dry ice, place the lid on the bucket, and allow the mixture to sit for 10 minutes to freeze the the temperature of the dry ice. At most, full freshly cut buds resting in dry ice take 30 minutes to freeze.
After freezing, vigorously shake the bucket. Alternate between shaking it up and down and swirling it around. Periodically check the bottom of the second bucket to see the quality of the trichomes.
Extraction and fraction quality.
Are the trichomes at the bottom of your bucket a light amber/golden color? Or are the trichomes now mixed with pulverized plant material?
You will likely want to collect a few different fractions – head fractions to heart fractions to tail fractions, just like a moonshiner. Shake a few times and look in the bucket. You might want to keep the first fraction for full melt hash (head fractions). Then shake vigorously and take a few more fractions (heart fractions). Finally collect the last fraction that will have the most broken up plant material in it (tail fractions). Now you have a range of grades (i.e. piles of different quality), ranging from golden trichomes to trichomes mixed in with plant material that have a green tint – heads through hearts through tails.
You can repeat this process several times over to collect all your grades as different fractions, but from multiple batches of fresh materials. Then you can pool them together and use them for individual batches in your extractor. Normally you pack your column with buds. Dry ice hash, or trichomes, just get poured into a smaller column and run as normal.
The goal here is to improve efficiency.
Less time running butane solvent through your closed loop extractor is a very good thing. It takes attention to detail every moment it’s running. One slip up, and you may lose your product or your life. From a risk based approach, Hemp Hacker believes this to be the very “best practice” that one can immediately implement in their extraction process.
The butane extraction side of the process works just like a normal nug run, but with an additional concentration step. While it adds a step on the front end, it reduces multiple steps on the back end. The main difference is that you concentrate the trichomes with dry ice in the first place, then dissolve them with butane. On a nug run, you would just be dissolving trichomes from buds with butane, and then collecting the cannabinoids and terpenes as an oil; the caveat is that you will have to do many more nug runs than with a trichome run.
Benefits of a 2 step extraction process.
While you may have green (tail) fractions that aren’t suitable for connoisseur grade hash, the plant material contamination will be filtered out by the filter plates at the bottom of your extraction column. This process takes care of the number 1 problem hash connoisseurs complain of – lack of potency due to plant material contamination in dry ice hash. You get the best of both worlds. An opportunity to collect the highest grade dry ice hash fractions with little to no contamination, and you also get to perform an extraction on the fractions that are contaminated with plant material and filter it out, yielding a high quality reduced wax extract.
Controlling humidity and water content during extractions is an important component to making quality extracts. When water contaminates an extract, it increases its tendency to auto butter when making shatter. This translates to reduced stability of an extract and a shorter shelf life. The two step process eliminates the largest source of water – the plant materials. The dry ice freezes all the water in the plant material, be it fresh frozen or crispy dry nugs, and allows the frozen glandular stalks that support the trichomes to be sheared off. The sheared trichomes fall off though the 220 micron bag and you have majorly reduced a source of water contamination (i.e. the plant materials).
Conclusion.
Using the two step process of dry ice hash extraction followed by butane extraction in a closed loop system is a drastic improvement of efficiency. It reduces the total number of runs required, the amount of butane that needs to be dehydrated, and the inherent risk of using butane and hydrocarbons as a solvent. Given those factors, turning your one step nug run extraction process into a two step nug to trichome process will greatly improve your efficiency and in turn, profits.
Concentration – the amount of something (in weight) in a given space (volume) – e.g. pounds/gallon, grams/milliliter.
Contamination – any impurity in product – e.g. plant cell wall debris, water in extracts.
Fractions – different grades of trichomes/hash depending on amount of plant material contamination.
Risk based approach – examining the inherent risks involved in a process and eliminating risks to improve the product safety or process safety.
As always, if you have any questions please post them in the comments section. Your questions and time are valuable and we will make every attempt to help you through your process.