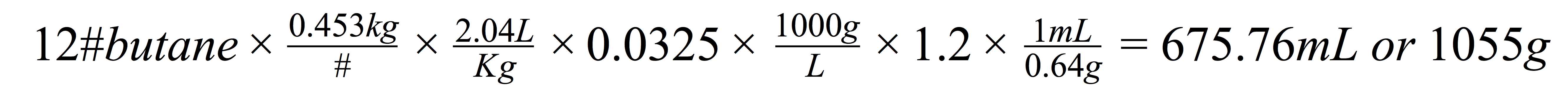https://patents.google.com/patent/US8530679B2/en?q=thc&q=butane&q=cannabis
In 2007, a patent was filed for delta-9 THC processing. It covered a range of organic solvents with boiling points preferably below 0C – ie “low boiling point solvents.” This fits the description of several hydrocarbons – the author preferred isobutylene, propane, butane, and cyclobutane.
This patent was validated with very pure THC – from 95-99% – nearly reagent grade. So while it validates the situation in under perfect laboratory conditions, it does not reflect the true extraction conditions that happen every day for medical and recreational extractions.
The important points of this patent are the temperatures. The desired solvent exists as a gas at room temperature, but can be in the liquid phase when put under pressure and temperature constraints. Controlled temperatures not only dictate the phase of the hydrocarbons (i.e. gas or liquid), but also prevent degradation of cannabinoids, preserve terpenes, and reduce solubility of plant lipids.
Some side notes on the choice of solvents were low toxicity, low environmental impact, and generally recognized as safe for use in pharmaceutical applications. These factors combined are some of the reason for using propane and butane as the industry standard for cannabis extractions.
That covers the theory behind the process. Below you’ll find a few points on the methods that make this a very good process.
Assuming one has an understanding of the standard closed loop extraction process, you can imagine how this process goes. You’ve passed your butane/propane through the extraction column and the extract is sitting in the collection vessel.
It does not specifically say in the patent, but there are two ways this could go. The first option is that the butane is boiled off (reclaimed) to a certain percentage, and then transferred to a secondary container. The second is that the butane is allowed to boil off without care for reclaiming. I find the second option less likely, but it is a possibility.
Assuming the first option, the partially purged extract is transferred to a second collection chamber while it is still of high enough viscosity. This process is called a cannula transfer. The second collection vessel is where there are inert conditions, and the butane/propane is evaporated off.
Solvent is evaporated by increasing the temperature of the second collection vessel and passing inert gas over the surface of the extract – in this case, I’m almost sure the author used a side-arm round bottom flask. I’ve covered the value of inert gasses in a few of my posts at Hemphacker, but it is definitely a standard operating procedure in a chemistry laboratory. In this case, they use argon gas to assist the removal of butane from the extract.
Using inert gas helps remove the solvent by changing the dynamics of partial pressures at the surface of the solution – this is a long topic and out of the scope of this article. However, the change in partial pressure increases the rate of evaporation for butane and allows one to to reduce the temperature to 4C, from what would normally be done at much higher purging temperatures. Now we’re in terpene preservation territory.
The low temperatures are beneficial because it reduces the degradation of the cannabinoids, and also keeps the terpenes in a low-volatility condition. In addition, the author claims that these temperatures make it easier to handle the extract.
As a result of evaporating off propane/butane at a lower temperature, the author claims that the invention induces a more crystalline form of the extract rather than the formation of a homogenous solid. I would hypothesize that the slow cool temperatures allow crystal formation, in what is a more gentle process that does not disrupt the nucleation of seed crystals. They claim to have shown this by x-ray diffraction, but no data is given to supplement the claim. Still, the extract is not 100% crystalline, as claimed by the author; as an analogy, think of how some parts of an extract will auto-butter before others.
One point to keep in mind is that this is a pure starting material, for the sake of explaining the invention of the process. By experience, a chemist performing extractions knows that the results vary by strain to strain, because of the different distribution of cannabinoids and terpenes.
This is a fundamental patent to understand the origins of live resin extractions. This author has several more patents on the subject, but I chose to write about this one because it was the “first mover.” The cannabis community owes much thanks to pioneers in science, who laid the way for us to preserve the ultimate essence of the plant.
I hope that you all find this interesting. Please share your thoughts and comments!