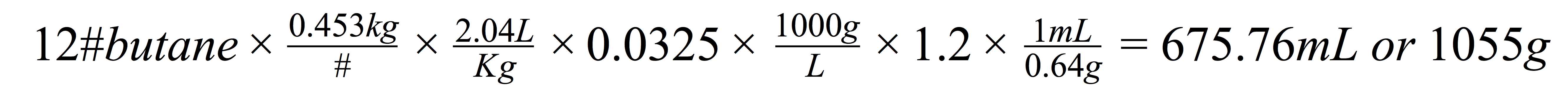Water kills butane hash oil yields
Butane picks up water during extractions and causes lower yields.
Butane picks up 3.25mL of water per liter and propane picks up 3.9mL of water per liter. To put it simply, water in butane and propane takes up space that prevents cannabinoids and terpenes from being fully extracted. That’s a problem.
How does water contaminate extractions?
Water contaminates extractions because of its effects on solubility – water in your butane and propane decreases the potential solubility of cannabinoids and terpenes, but it also picks up water soluble contaminants like plant chlorophyll, alkaloids, and flavonoids. If you’re looking for the simplest solution, just dehydrate your butane/propane. If you’re looking for perfection, purge your extractor with CO2.
Improve yields in 2 simple steps.
It’s all about the chemistry of inert conditions/reactions. There are two simple steps for high quality live resin extractions: 1. dehydrate your solvents; 2. purge your live plant materials and extractor with a dry gas like CO2. Putting together the steps of dehydrating your butane and purging your extractor will most certainly increase your yields, but it also functions to reduce contamination.
Dehydrating butane and propane is the most important step to improve yields in live resin and regular extractions.
Dehydrating your solvents is as easy as packing your extraction column with a substance such as 3A molecular sieves or activated alumina. Molecular sieves and activated alumina are used to dehydrate butane/propane in the gas phase – ie you do not pass liquid butane/propane through a column packed with these desiccants.
3A Molecular sieves are widely employed in the lab setting, where they’re used to dry solvents or keep solvents dried in the first place – they can hold up to 19-20% of their water weight. Molecular sieves are also FDA Approved for direct contact with consumable products. Activated alumina has a higher water capacity. It’s used in number of industrial drying applications for hydrocarbons, but there isn’t sufficient data proving its safety beyond “Satisfactory” compatibility with butane and propane.
Check out the post about recovery pump setup and see how to set up a system that will effectively dehydrate your butane and propane.
Desiccant math made easy.
You need to do little math to figure out how much space your molecular sieves will take up, and to figure out how many grams of molecular sieves you need. Molecular sieves cost approximately $100/500g, but they are nearly infinitely reusable. You can regenerate or dehydrate them by heating them up in a vacuum oven and pulling full vacuum. Here’s the math:
Most extraction artists use US pounds to measure their butane, so we have to do a few conversions. Let’s say you have 12 pounds of butane. You need to convert it to kilograms, so you can take into account the density of butane (assume 1bar/14.5psi and 20C/67F); you use the density to convert the mass (i.e. weight) of the butane into a volume (liters). Now you can multiply the number of liters by the water:butane conversion factor to determine the amount of water that the 12 pounds of butane can hold. Finally, multiply the amount of water in your butane by the water capacity of the molecular sieves. This shows you minimum number of milliliters or grams of molecular sieves you need to dehydrate your butane. That said, 675mL of molecular sieves weighs approximately 1000g. Buy two 500g containers and you’re home free.
Conclusion.
Dehydrating your butane is a step forward in improving your extractions. Not only does water in butane decrease extraction efficiency, but it also causes increases the extraction of plant contaminants like chlorophyll, alkaloids, and flavonoids. If you’re looking for perfection, you’re going to run your extractor like an organic chemist synthesizing a compound under inert conditions. You’re drying off all the water from the extractor walls with a hot air gun, then you’re pumping out the water trapped in the atmosphere by pulling a full vacuum. These steps make for a higher quality and more consistent product.
Resources:
Thermodynamic properties of butane and propane:
- http://encyclopedia.airliquide.com/encyclopedia.asp?GasID=8
- http://encyclopedia.airliquide.com/encyclopedia.asp?LanguageID=11&CountryID=19&Formula=&GasID=53&UNNumber=#MaterialCompatibility
- http://www.nist.gov/data/PDFfiles/jpcrd331.pdf
Activated alumina:
Molecular sieves:
As always, if you have any questions please post them in the comments section. Your questions and time are valuable and we will make every attempt to help you through your process.