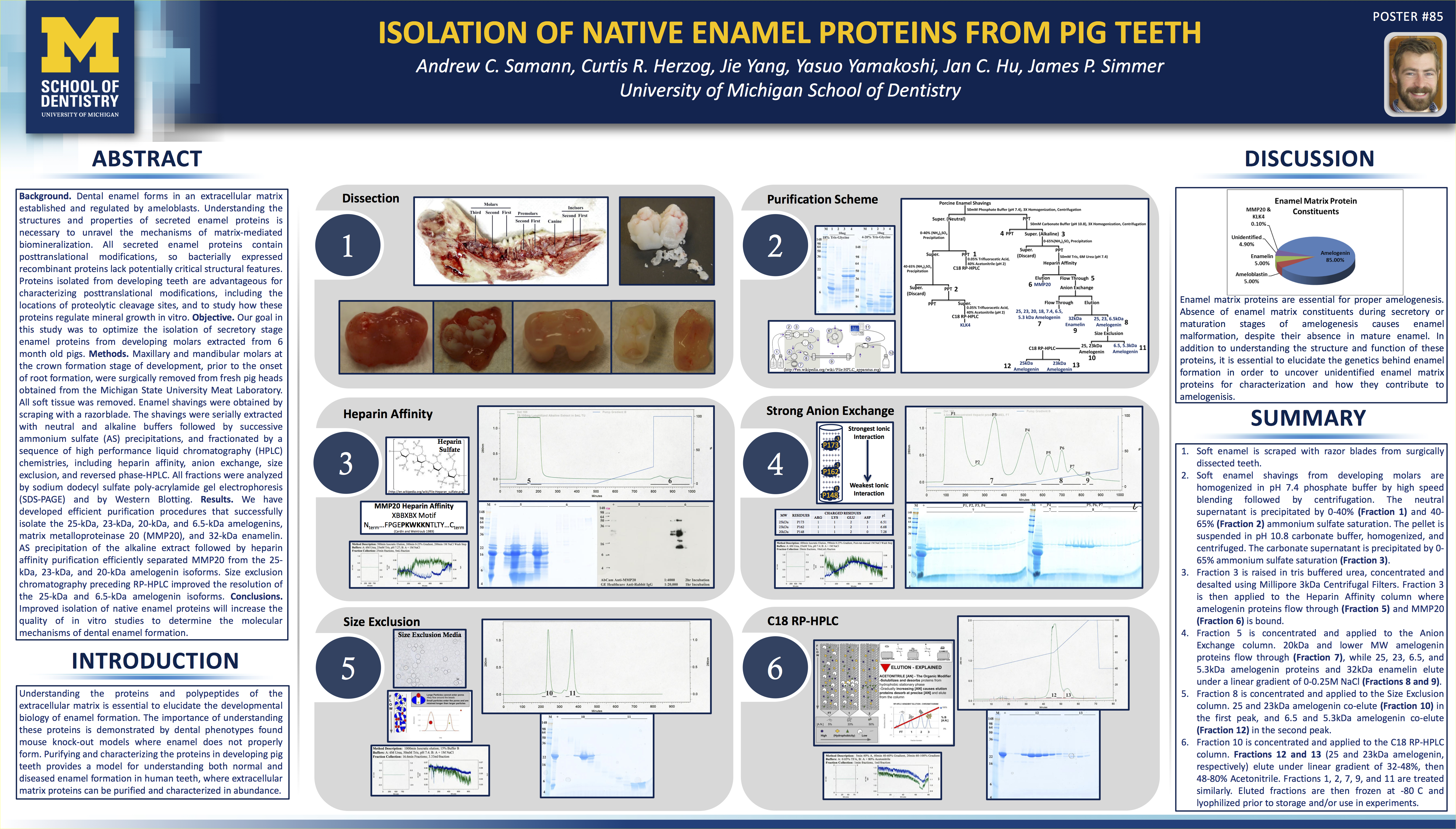
The Code of Federal Regulations Title 21, Part 210 is dry reading, but it’s necessary for the cannabis industry to digest. Understanding these regulations, despite their dry nature, is the job of the Quality Assurance unit. In fact, it’s optimal that everyone involved in cGMPs is very aware and familiar with this documentation.
For the sake of this writing, my aim is to inform quality assurance (QA) and quality control (QC) personnel of what they need to know. This is a good place to start. You must understand the following definitions in order to read further into the literature of Good Manufacturing Practices.
The 21 CFR 210 and 211 only describe the minimum current good manufacturing practices.
The 21 CFR describes the minimum methods, facilities, and controls that need to be in place for manufacturing, processing, packing, and/or holding any drug product. The main goal is to make sure that drugs are manufactured to the specifications they claim to have – it ensures:
- Safety
- Identity and strength
- Quality and purity characteristics
As usual, there’s a consequence for not following the rules, which can be very costly. If it’s found that a company manufacturing under cGMPs is not complying with the 21 CFR, they may:
- Determine the drug is adulterated
- Hold the person responsible who was in charge of the process
When your company is found to be violating the 21 CFR, you might get a publicly published 483 warning letter that will say something along the lines of:
“You should take prompt action to correct the violations cited in this letter. Failure to promptly correct these violations may result in legal action without further notice, including, without limitation, seizure and injunction.”
Right now, cGMPs for the cannabis industry are in their infancy. When the FDA is regulating the industry, it will be a different story.
Definitions
When you fully understand these terms, you will have a much easier time understanding the 21 CFR part 211. It’s the baseline of information that sets the stage for everything that’s to come. Take warning though – these are complicated definitions, and often require a background in chemistry or the sciences to fully understand.
Some definitions will be followed by an explanation tying it into terms related to the cannabis industry, as necessary.
Batch – a specific quantity of a drug or other material that is intended to have uniform character and quality, within specified limits, and is produced according to a single manufacturing order during the same cycle of manufacture.
This could be a batch of plants that finished their flowering period at the same time, and were harvested at the same time. Alternatively, it could be the finished extract that was produced by one cycle of a CO2 or hydrocarbon extraction system.
Component – any ingredient intended for use in the manufacture of a drug product, including those that may not appear in the final drug product.
This could be the CO2 or butane used in an extraction.
Drug Product – a finished dosage form, for example, tablet, capsule, solution, etc., that contains an active drug ingredient generally, but not necessarily, in association with inactive ingredients. The term also includes a finished dosage form that does not contain an active ingredient but is intended to be used as a placebo.
This could be flowers that have been fully processed to their dried and cured form, ready for use. It could also be an extract that has been fully purged, packaged, and labeled, ready for use.
Fiber – any particulate contaminant with a length at least 3X greater than its width.
Nonfiber releasing filter – any filter, which after appropriate pretreatment such as washing or flushing, will not release fibers into the component or drug product that is being filtered.
Active ingredient – any component that is intended to furnish pharmacological activity or other direct effect in the diagnosis, cure, mitigation, treatment, or prevention of disease, or to affect the structure or any function of the body of man or other animals. The term includes those components that may undergo chemical change in the manufacture of the drug product and be present in the drug product in a modified form intended to furnish the specified activity or effect.
In the case of cannabis, this would be all the cannabinoids that are present in the final product. Cannabis is tricky in that its final forms are usually a mixture of cannabinoids, and not pure product. All together, the mixture of cannabinoids can be considered the active ingredient.
Inactive ingredient – any component other than an active ingredient.
In the case of cannabis flowers, it would be everything but for the cannabinoids. In the case of an extract, there can be waxes and other lipids that are present with the active ingredient.
In-process material – any material fabricated, compounded, blended, or derived by chemical reaction that is produced for, and used in, the preparation of the drug product.
In general, there are purification steps involved in producing cannabis products, and relatively few chemical reactions.
Lot – a batch, or a specific identified portion of a batch, having uniform character and quality within specified limits; or, in the case of a drug product produced by continuous process, it is a specific identified amount produced in a unit of time or quantity in a manner that assures its having uniform character and quality within specified limits.
Similar to a batch, but is the result of a continuous process. Therefore, a lot would be the packaged components from, for example, 100 bottles filled with cannabis tinctures. The tinctures would be filled in a continuous process and all 100 would be filled in a specific identified amount of time or quantity.
Lot number, control number, or batch number – any distinctive combination of letters, numbers, or symbols, or any combination of them, from which the complete history of the manufacture, processing, packing, holding, and distribution of a batch or lot of drug product or other material can be determined.
This is the identifying number of a lot or batch. It’s a locally produced number that’s used to track all activity that was associated with manufacturing a drug substance.
Manufacture, processing, packing, or holding of a drug product – includes packaging, labeling operations, testing, and quality control of drug products.
Quality control unit – any person or organizational element designated by the firm to be responsible for the duties relating to quality control.
Strength
- The concentration of the drug substance (e.g. weight/weight, weight/volume, or the unit dose/volume basis).
- The potency, i.e., the therapeutic activity of the drug product as indicated by appropriate laboratory tests or by adequately developed and controlled clinical data (e.g. expressed in terms of units by reference to a standard).
Theoretical yield – the quantity that would be produced at any appropriate phase of manufacture, processing, or packing of a particular drug product, based upon the quantity of components to be used, in the absence of any loss or error in actual production.
Actual yield – the quantity that is actually produced at any appropriate phase of manufacture, processing, or packing of a particular drug product.
Percentage of theoretical yield – the ratio of the actual yield (at any appropriate phase of manufacture, processing, or packing of a particular drug product) to the theoretical yield (at the same phase), stated as a percentage.
Acceptance criteria – the product specifications and acceptance/rejection criteria, such as acceptable quality level and unacceptable quality level, with an associated sampling plan, that are necessary for making a decision to accept or reject a lot or batch (or any other convenient subgroups of manufactured units).
Representative sample – a sample that consists of a number of units that are drawn based on rational criteria such as random sampling and intended to assure that the sample accurately portrays the material being sampled.
If you have more questions, check out www.oriongmp.com and get a free consultation on putting together your Cannabis related Good Manufacturing Practices and Quality Manufacturing Systems.