This question was asked in the comments section in cannabis extracts in Reddit – how do you produce pure Δ9 THC that’s appearing in dispensaries??? A fellow named Adam Mueller answered this question, and figured out the sub/supercritical conditions to produce pure Δ9 THC and was issued a patent for it last year.
It is also possible to extract and purify THC to a high concentration using other methods. However, they mostly use solvents that are known to be carcinogenic. CO2, on the other hand, defines a “Green Solvent” – no carcinogenicity, and no waste products that pollute the environment.
Adam Mueller has several patents describing the extraction of cannabinoids from hemp and drug varieties of cannabis. He represents Delta-9-Pharma GmbH, a company out of Germany. While this information may not be novel to people who are already performing extracts, it is of interest to people who would like to learn about the conditions.
This patent explains the steps from taken from grinding up plant matter, subjecting it to subcritical or supercritical conditions and producing a pure CBD and THC product. An interesting point is that CBD can be converted to THC with the right conditions. Past that, the product is dewaxed and ready for delivery in the activated decarboxylated form.
There are some aspects of the patent that are very useful, but there are downsides to running supercritical CO2, given what we know about the “entourage effect” described by Ethan Russo (1). Cannabinoids alone do not have the highest medicinal benefits as a mixture terpenes and cannabinoids. While this patent describes a way to obtain highly purified CBD and THC, the terpenes end up in a separate fraction. Recombining THC, CBD, and terpenes is not unheard of, but it does require a bit more work.
Raw materials
Mueller’s primary source of raw materials is hemp. This is a logical starting material for countries where it is illegal to cultivate marijuana. For the validation of the patent, he used five different strains that are from French, Hungarian, and Finnish origins. In general, these are industrial hemp varieties that are used for fiber production. The legal requirements for such strains are that they contain no more than 0.3% THC by dried weight of the starting materials.
Sub/Supercritical conditions
Mueller provides a range of conditions that can be used to extract cannabinoids with supercritical CO2. Supercritical conditions range from 31-80℃ and 75-500 bar. Subcritical conditions range from 20-30℃ and 100-350 bar.
Subcritical and supercritical extracts do not come out with the same consistencies. Subcritical extractions preserve more terpenes, while supercritical extractions lose terpenes and increase the waxes. Subcritical extracts have an oil like consistency, have less plant lipids (waxes), and may not need dewaxing, depending on the application and what customers want – it is nearly ready for use in vape pens or to be dabbed. Supercritical extracts have a more solid “crumble” consistency, have more plant lipids, and most certainly require dewaxing in order to be used in vape pens or dabs. Both can be used for edibles.
A follow up point is that both subcritical and supercritical runs can be done on the same starting material. First perform a subcritical run and collect the extract – the extract will still have some terpenes present without being overwhelmed with waxes. Then, the same starting material can be run a second time under supercritical conditions. Running subcritical conditions produces relatively low yields, so to maximize yield (i.e. profitability) one really needs to run supercritical conditions. You may find the extra work of doing two runs is not worthwhile, considering you have to have to do twice the work. There’s always a trade-off somewhere in a process, and this one is for you to decide. For maximum yield, Mueller suggests performing two runs on the same material.
Mueller increases yields by using “entraining agents.” Butane, propane, and ethanol are used in concentrations of 1-10%.
Entraining agents have different properties than supercritical CO2. The critical point (CP) of CO2 is 73.8 bar and 31.5℃; butane has a CP of 38.0 bar and 152℃ (2); propane’s CP is 42.5 bar and 96.7C (3); ethanol’s CP is 63 bar and 241℃. A mathematical description requires computational chemistry to show how the entraining agents interact with the CO2. Suffice it to say, CO2 will be in the supercritical phase and the entraining agents will be in the liquid phase (3), (4). This changes the solvent characteristics of the CO2 and improves extraction yields.
His optimal supercritical conditions range from 45-65℃, 100-350 bar, with his best conditions being 60C and 250 bar. His preferred subcritical conditions range from 20-30℃ and 100-350 bar.
Mueller illustrates the system in figures 1-3. It’s a complicated system that is appropriately named a “CO2 extraction plant.” This system was designed for process scale. The system has three components: the extraction system (figure 1), the CBD cyclization system (figure 2), and the CBD/THC separation system (figure 3).
How CO2 supercritical work – solubility
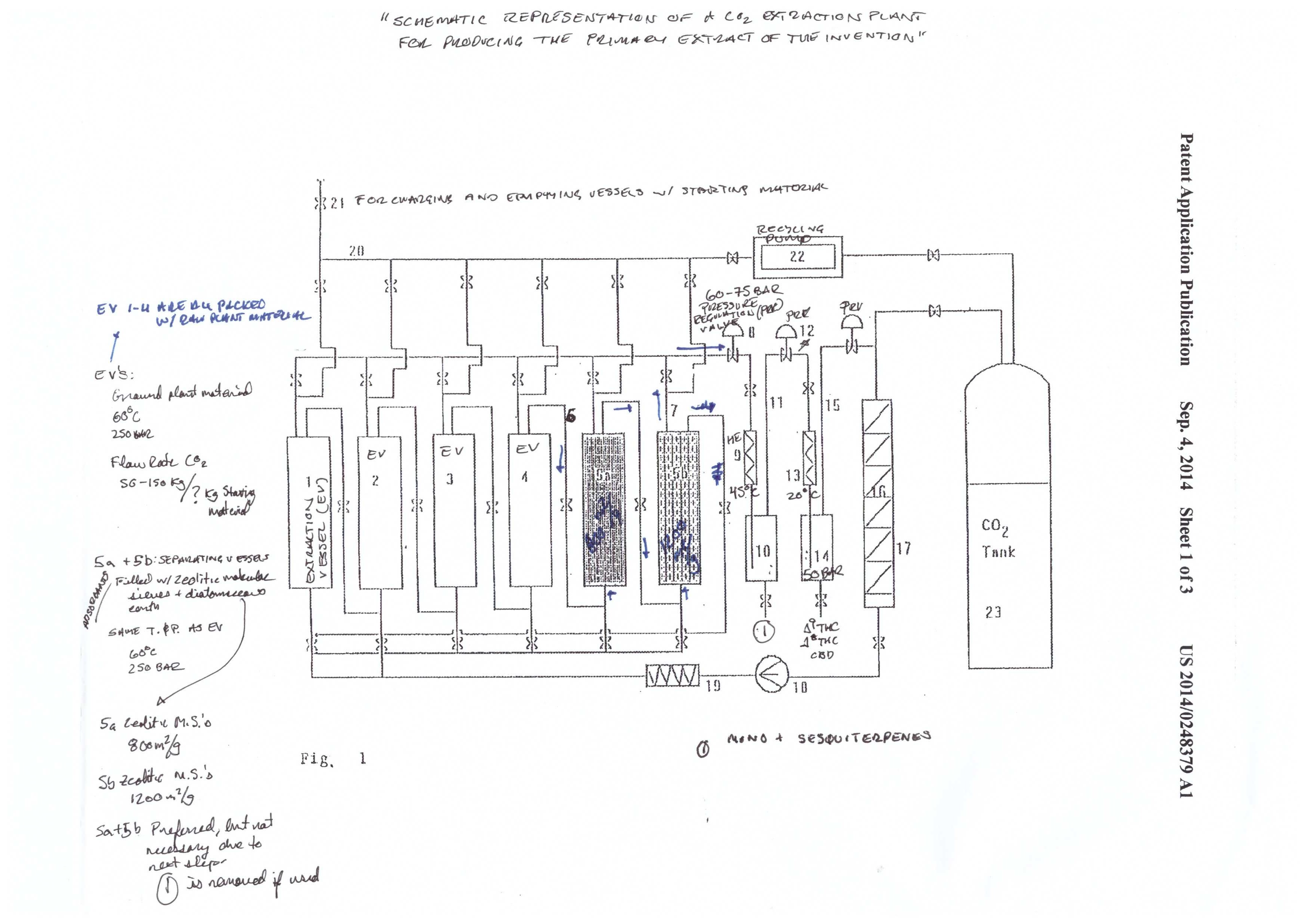
The extraction starts in the extraction vessel (F1: 1-4). The column(s) is packed with plant material, and the sub/supercritical CO2 begins to strip the plant material of cannabinoids. The cannabinoids are called the solute, and the CO2 is called the solvent. A solvent becomes saturated when it has no more “room” for more solutes to be carried by the solvent.
The cannabinoid saturated solvent then passes into the separating vessels (F1: 5a-5b) in a continuous process. Fresh solvent enters the extraction vessel, dissolves cannabinoids and other phytochemicals, and carries them into the separating vessels. The cannabinoids are the first solutes to pass through the separating vessels, followed by terpenes, and then the undesirable phytochemicals.
The order of the solutes entering the separating vessel (packed with adsorbents) is dependent on how strong of an interaction each molecule has with the adsorbent media. Cannabinoids have the weakest interaction (first out) and the undesirable phytochemicals have the strongest (last out).
The solubility of the different solutes in the solvent depends on both pressure (P) and temperature (T). The general scheme of the system is to go from high P to low P, and high T to low T, in graduated steps. At high P and T have the highest solubility.
As the P and T are reduced, the solubility of certain components is reduced. For example, the extraction vessels and separating vessels run at 60℃ and 250 bar. The terpene/cannabinoid rich solvent is then pumped over to the first collection vessel (10), at 45℃ and 60-75 bar. That reduction in P and T causes the terpenes to fall out of solution. Meanwhile, the cannabinoids are still in solution.
The cannabinoids are then pumped to the next collection vessel (14). T and P are reduced to 20℃ and 50 bar. Under these conditions, the cannabinoids fall out of solution, and are ready for collection.
Dewaxing and decarboxylation
Dewaxing is pretty standard in the industry these days. Unless you run very cold conditions, you’re going to pick up wax in both hydrocarbon and CO2 extractions. To put it simply, cold ethanol is used to dissolve the extract, followed by freezing and filtration. The extract can then be moved on to decarboxylation, which is a well documented process in the industry.
Converting CBD to THC
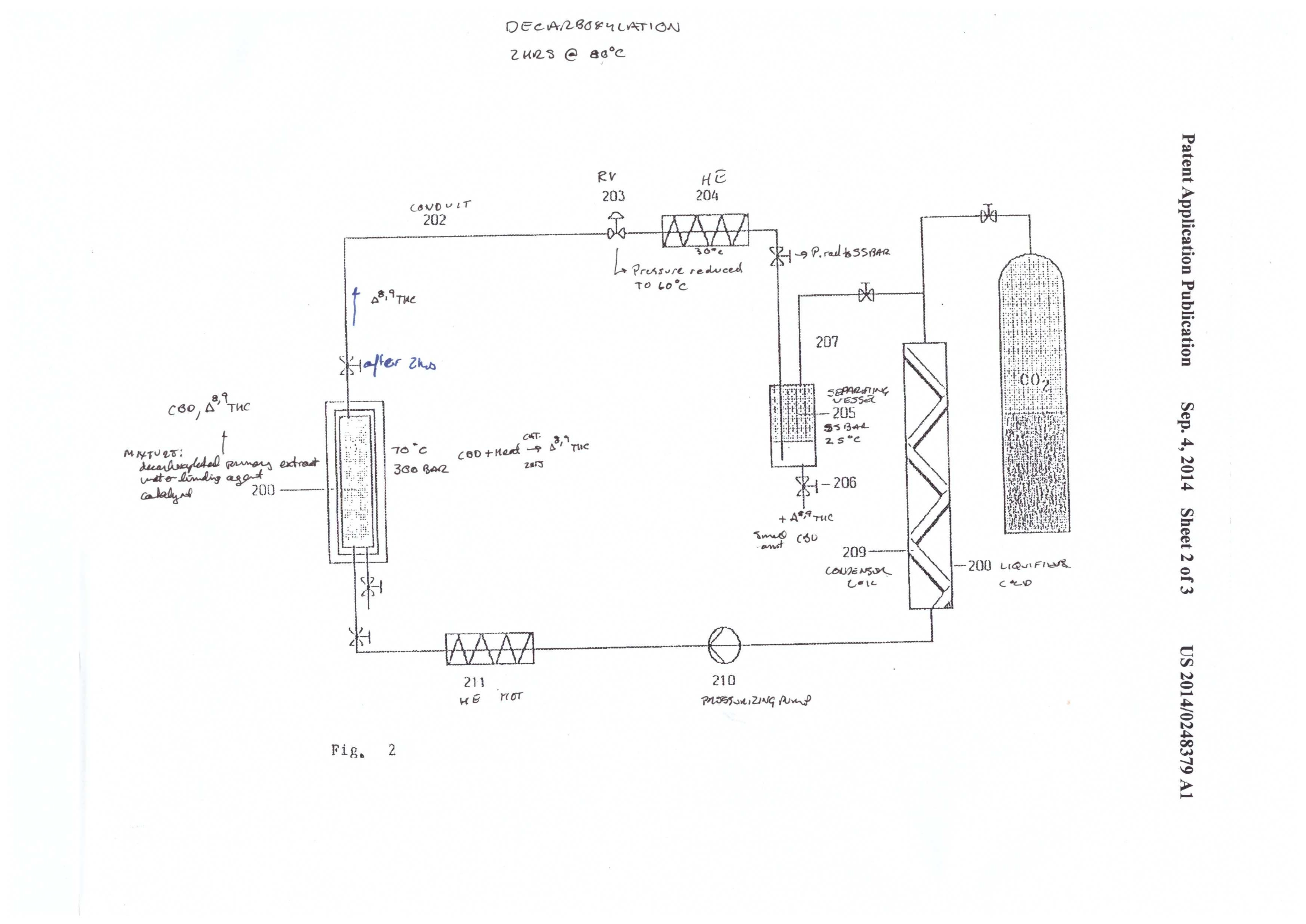
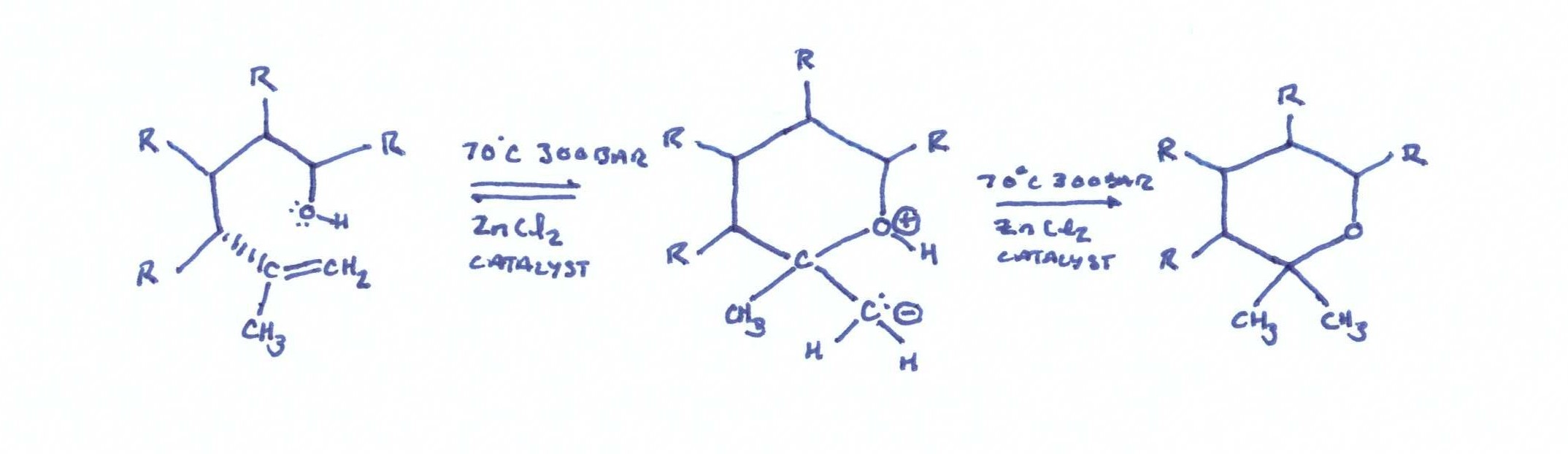
The second admirable part of this patent is the cyclization reaction of CBD to THC. Molecular/zeolytic sieves and zinc chloride are employed as a catalyst to aid the reaction. The molecular sieves act as a water binding agent, and the zinc chloride acts as a catalyst to reduce the activation energy required for the cyclization reaction.
The conditions are 300 bar and 70℃, and the reaction takes place over a 2 hour time period. The apparatus in figure 2 shows a simple reaction vessel containing the catalysts and extract that are plumbed to a separate collection vessel. The extract is pumped out to the collection vessel by precipitation with 55 bar and 25℃.
Pure Δ9 THC
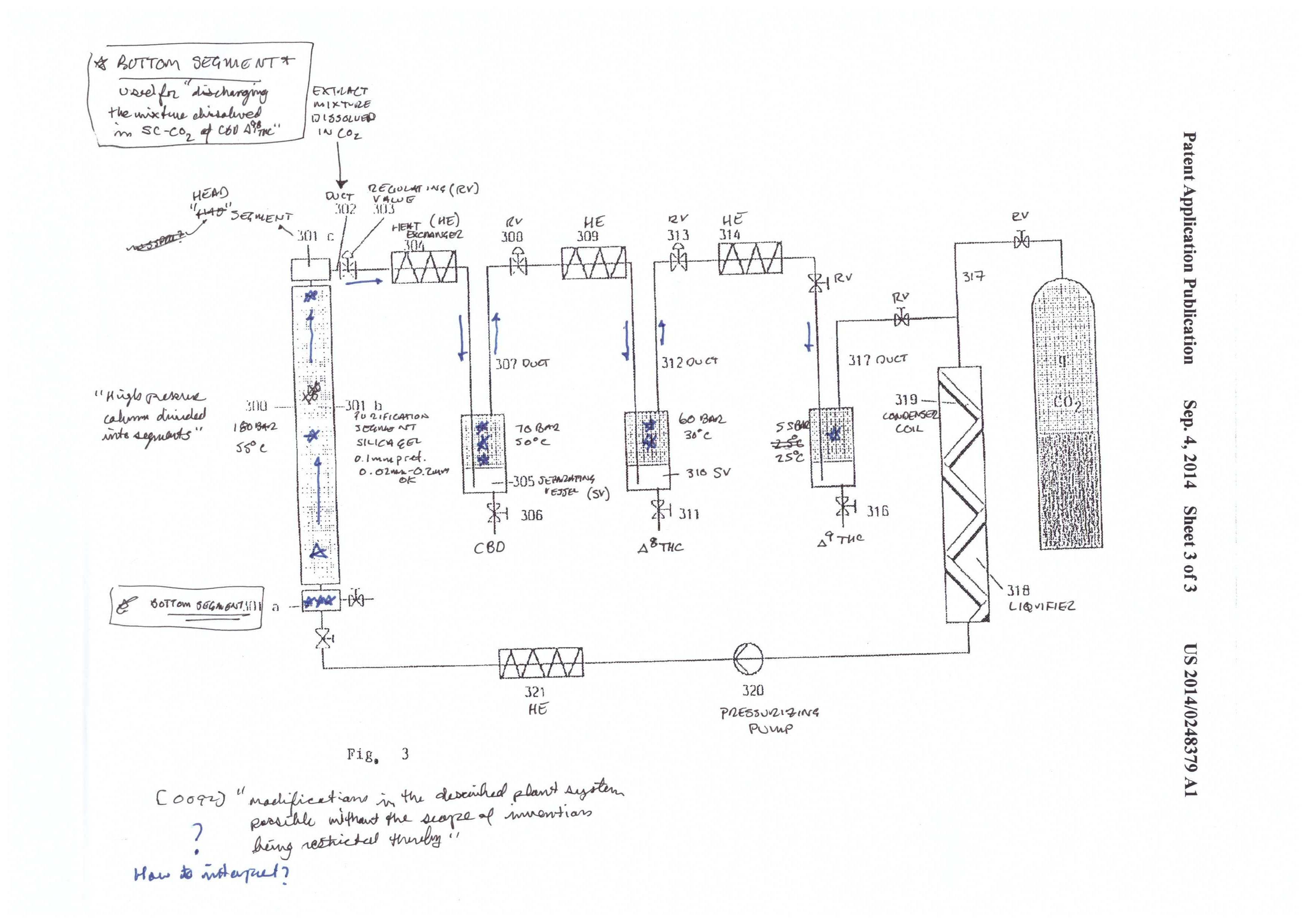
The final step described in the patent is the separation of CBD, Δ8 THC, and Δ9 THC. This is achieved by using a purification material/media commonly used in separation science – silica. As is described below in the chromatography, silica has a charge to it, that reacts with the molecules that are to be separated/purified from one another. Some molecules have a stronger interaction than others and therefore travel slower through the silica packed column. In this case, Mueller ends up with pure fractions of the three cannabinoids.
The silica used in the patent has an average size of 0.1mm, and is commonly used in separation science. Looking at figure 3, you will see that the CBD/THC mixture starts at the bottom of the column. When the supercritical CO2 is pumped into the system, the mixture travels up the column and begins to separate the mixture.
After the separation column, there are three collection vessels. As with the initial extraction, CBD, Δ8 THC, and Δ9 THC are separated out by precipitation. The precipitation occurs from dropping the P and T in steps from high to low. CBD is precipitated at 70 bar and 50℃. Δ8 THC is precipitated at 60 bar and 30℃, and Δ9 THC is precipitated at 55 bar and 25℃.
Separation of plant phytochemicals, terpenes, and cannabinoids
The first step in this patent produces pure THC/CBD while removing the plant alkaloids, flavonoids, and chlorophyll. It also removes terpenes.
Without good separation/purification, the terpene fraction can be contaminated with plant alkaloids, flavonoids, and chlorophyll. This is one of the inherent downsides to CO2 extractions compared to butane extractions; phytochemical contamination can be reduced in butane extractions by keeping conditions at sub-zero temperatures. In CO2 extractions, it can be reduced by not cranking the extractor into “hyperdrive” conditions.
Although the loss of terpenes is an inherent problem with supercritical CO2, it’s easiest to preserve terpenes by performing a subcritical run followed by supercritical run. This maximizes the yields, but is not explicitly described as the method used in this patent – it only suggests that the materials are extracted a second time.
Adsorbents
The use of adsorbents is illustrated by way of a packed column in figure 1. An adsorbent is a substance that attracts molecules, and the molecules subsequently adhere to the surface. Note that this is not the same as absorbing, where a molecule is taken into the structure of the substance. An adsorbant has a transient interaction with the molecules it attracts, where the molecules stay on the surface. An absorbant actually soaks up a molecule into its pores, rather than just staying on the surface.
Adsorbents are used in this patent to remove undesirable molecules, such as alkaloids, flavonoids, and chlorophyll. The adsorbents attract undesirable molecules. They adhere to the surface under sub and supercritical conditions, temporarily falling out of the supercritical solution, while the terpenes and cannabinoids stay in solution and pass on to the next section of the system. It is a clever way to remove the undesirables, but is relatively common in separations science.
The adsorbents are silica gel, diatomaceous earth, bentonite, bleaching earth, activated carbon, and mixtures of magnesium oxide and alumina zeolitic. Although it’s not explicitly said, there are two ways that the adsorbents can be used. First, you can pack the plant matter on top of a bed of adsorbents. The second method is to have secondary column in-line, downstream of the extraction chamber, that pulls out all undesirables. The second is much more clean and efficient, but requires additional equipment, and at these kinds of pressure ratings, stainless steel is not cheap.
Chromatography
A more subtle point, that may not be immediately apparent, is the application of chromatography. Chromatography is loosely defined as the separation of mixtures. In this case, the mixture to be separated is the undesirable plant phytochemicals and the desirable terpenes/cannabinoids. The main function of chromatography media (i.e. adsorbents and silica) is to allow some molecules to travel through the media faster than others.
When a molecule has a strong interaction with the chromatography/purification media, it travels slower. When a molecule has a weak interaction, or is repelled by the media, it travels faster. Imagine each phytochemical being a molecular magnet. For example THC and CBD are very weak magnets compared to alkaloids, flavonoids, and chlorophyll. In fact, the undesirable molecules are like strong magnets.
The way this works, is that the adsorbents are also like strong magnets, but have the opposite charge of the undesirable phytochemicals. Opposites attract, and the undesirable phytochemicals are strongly attracted to the adsorbent, while the desireable terpenes and cannabinoids have a weak attraction to the adsorbent. The weaker the interaction, the faster the molecules can travel through the media, and vice versa.
Extraction, purification, and resolution in supercritical extractions
Extraction is the first step in any purification scheme. The industry is currently focusing on whole plant extracts, without much purification beyond dewaxing. This patent utilizes purification in the process – this is how CBD, Δ8 THC, and Δ9 THC are separated from each other.
As described above, chromatography is the mechanism of separating molecules. This can be done by columns packed with silica, and other packing materials. In chemistry, the packing materials are called purification media. Whatever packing material is used, the purpose is to slow down some of the molecules in order to separate the ones you want from the ones you don’t want.
You can’t talk about chromatography without talking about resolution. Resolution can be simply defined as the amount of separation of two molecules in a purification process. Depending on what kind of packing materials used, you can separate groups of molecules from one another. Some packing materials work better for some types of molecules, and is a chore to find the right media when starting a new purification scheme.
When you put together the extraction step and follow it with purification steps, you can separate groups of molecules. The choice of using purification is one that is dependent on whether one wants a pure product.
The end product
One really needs to ask themselves how important purity is. The more pure of a product desired, the more effort that is required. Do you really want pure crystalline THC and CBD? If yes, this process may be right for you. If you just want an extract, you may want to consider these steps, but take what information is useful to you.
Again, a process is only useful if it is economically viable. The extra effort spent in making pure products is significant, but is a daily reality in the pharmaceutical industry. A point to consider is that the closer you get to a pure product, the higher the chance of losing your product due to mistakes.
Having spent years in chemical purifications, plenty of solvent and product have been lost to simple, avoidable mistakes. To avoid mistakes, clearly define your process before you begin. Always have a plan and think about what you’re going to do before you execute the plan. Be careful, take your time, take good notes, and always keep the safety of the consumer as your top priority.
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3165946/clopedie/VaporPressureGraph/Propane_Vapor_Pressure.GIF
- http://encyclopedia.airliquide.com/images_encyclopedie/VaporPressureGraph/Butane_Vapor_Pressure.GIF
- http://encyclopedia.airliquide.com/images_encyclopedie/VaporPressureGraph/Propane_Vapor_Pressure.GIF




