This short article describes the theory and basics of Cannabis Chromatography. CannaChemist has spent quite a bit of time patiently waiting for molecules to separate in chromatography columns used to purify organically synthesised columns, and shares her knowledge here to help you resolve products like THC from CBD rich tinctures.
Chromatography
Chromatography, or “color writing” is a reliable purification and analytical technique that has been used for over a century. In 1897, the American chemist David Talbot Day observed that crude oil turned into bands of color as it seeped upwards through clay. In 1900, the Russian-Italian chemist Mikhail Tsvete used chromatography for the separation of plant pigments such as chlorophyll (green), carotenes (orange), and xanthophylls (yellow). Chromatographic techniques continued to advance substantially, gaining widespread use and winning the 1952 Nobel Prize in Chemistry. Today, there are countless variations of chromatography and even automated instruments that are used by scientists around the world, making all kinds of research more efficient than ever.
It’s all about sand… Silicon Dioxide.
Chromatography is a way of separating and purifying all chemical compounds, not just colors, taking advantage of their differences in properties. More specifically liquid, column, or flash chromatography is a practical and straightforward way to purify a substance from a complex mixture (a natural extract or chemical reaction) by passing it through an inert solid called silica, or silicon dioxide. The different compounds in the mixture will have different degrees of attraction, or stickiness, to the silica, and will pass through it at different rates.
Silicon dioxide, a major constituent of sand, is a network of silicon oxygen bonds. The less polar, or more oily compounds will have less attraction to the polar silica, and come off of the column first. If you had a mixture of THC and CBD, the THC would elute first, since it contains only one alcohol functional group instead of the more polar CBD, which contains two. During this process, the compounds and the silica are dissolved in solvents such as pentane and ethyl acetate. The ratio of ethyl acetate to pentane is increased during the column to elute compounds of increasing polarity. However, this practice can be problematic for greasy nonpolar compounds such as terpenes, and even cannabinoids. If there is not enough attraction between the molecules and the silica, they will all travel quite quickly and elute together, not achieving a good separation.
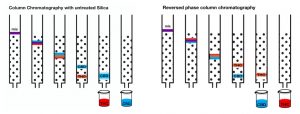
Reversed Phase Chromatography.
Another form of chromatography, called reversed phase, can be used for these instances. A special hydrocarbon-coated silica is used, which reverses the elution order. Polar solvents such as water and acetonitrile (an organic solvent) are used. The ratio of acetonitrile to water is gradually increased during the run to draw the nonpolar compounds through the stationary phase. THC has greater attraction to the hydrophobic stationary phase, so CBD travels more quickly. If you are looking to remove lesser amounts of THC from a predominantly-CBD sample, reversed phase chromatography, although more expensive than untreated silica, is an ideal technique.
Contact Us.
If you’re interested in learning more about chromatography and separating cannabinoids, send us an email at info@oriongmp.com.
This content was written and supported by Orion GMP Solutions.
