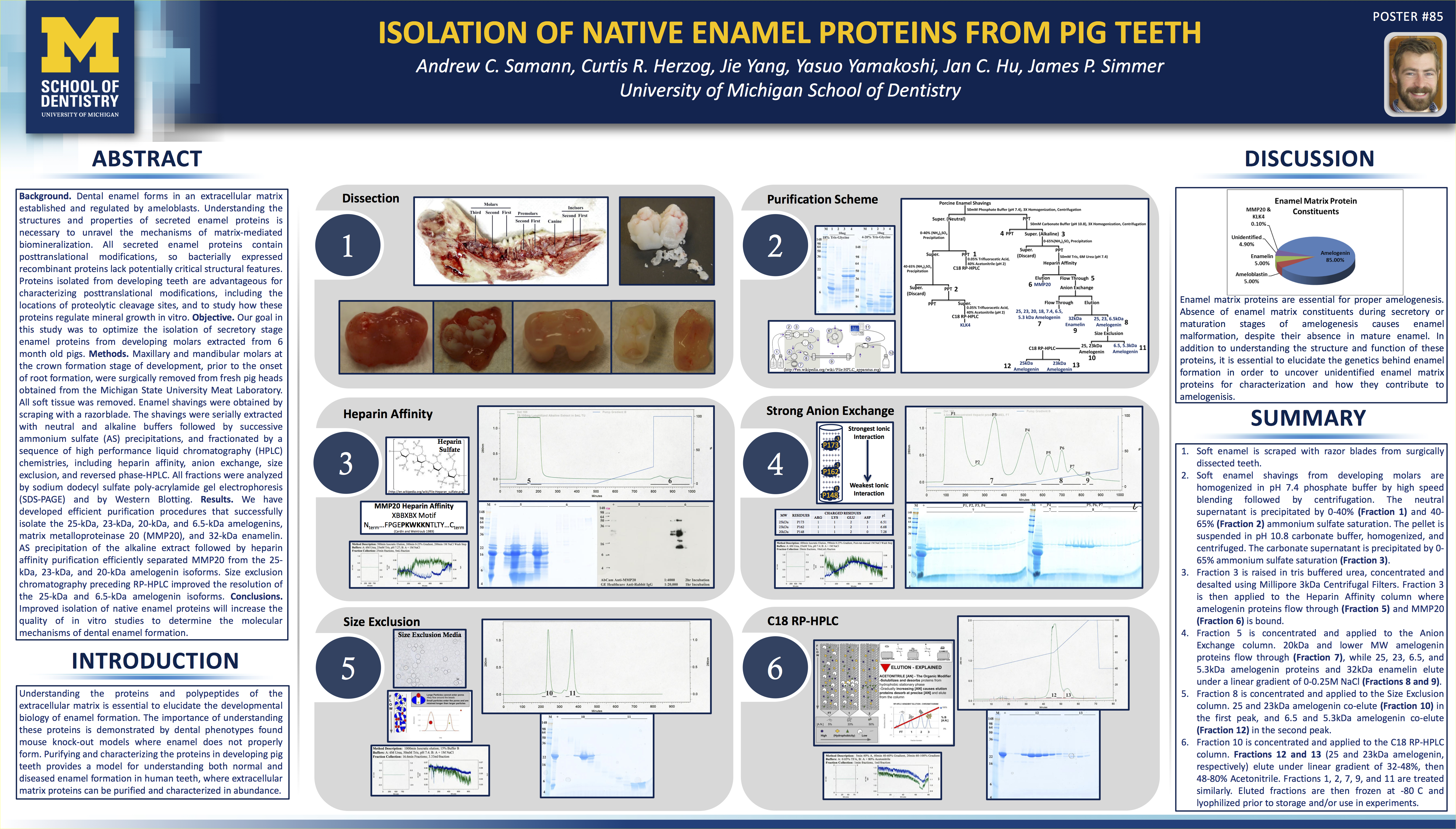
It’s always a pleasure when I get an email from someone asking how to break into the industry. I can appreciate the feeling – I was once there. I had hustle, and always worked hard, but I didn’t have a clear vision of the end game.
Ultimately, building yourself up in any industry requires experience. You dig into the work and make a name for yourself. There are many ways to get there, but it’s usually a nonlinear process.
I got my start in the industry in an unlikely place – as a Sergeant in the Marine Corps. I realized during my last tour in Iraq that the cannabis industry was in my future. I had my own personal reasons that drove me towards it, but I saw things lining up. I was honorably discharged in 2006, and I immediately got to work on my education in both cannabis and chemistry.
I hadn’t taken a math class in 5 years, and I had no real background in the sciences. Despite that, I started from the bottom and worked my way through all the liberal arts, math, chemistry, and biology courses. I hustled, and my work paid off with the rewards of leading chemistry study groups – I found that teaching is one of the most rewarding things I can do.
I attended the University of Michigan where I studied Biochemistry and spent my free time learning about the physiology of the endocannabinoid system. I wanted to learn everything about how cannabinoids affect the body and their therapeutic potential. I graduated with my B.S. in 2011 and tasted the accomplishment of my hard work. I planned on going through to a PhD program in Biomedical Sciences, but I first wanted to solid foundation in scientific research before jumping into it.
That’s where some luck comes into play. I landed a job in a biochemistry/genetics laboratory at the University of Michigan where I had the best mentors a young scientist could have. I had all the tools of the trade for HPLC, column chromatography, mass-spec, and a project that needed me to use all of them. I was a protein chemist. Every purification started with extractions, and moved on through multiple steps of column chromatography that ended with HPLC purification.
I scaled up processes and thought of myself as the Henry Ford of protein purification… Perhaps it was grandiose to think that way. Nonetheless, it’s where I learned to apply the scientific method on a daily basis, and I where I got my basic understanding for extracting and purifying compounds.
I found that a career in academic science was not for me. It is a surprisingly political atmosphere, and I’m not one for bickering. I was accepted into a PhD program, but dropped out just days before the program started. I knew it wasn’t right for me, and, besides, I had an awesome job in the pharmaceutical industry as a Good Manufacturing Practices Quality Control Chemist. It was there, that I realized Good Manufacturing Practices (GMPs) are the future of the cannabis industry – I finally had my clear vision of the end game.
I always kept myself busy moonlighting in the industry while working as a chemist by day. I put the two together, and found that my best bet was to share information and help other people. HempHacker has become my means of teaching people about different aspects of the industry that aren’t fully covered elsewhere.
Since my last job as a GMP QC Chemist, I’ve been doing GMP Consulting for the Cannabis industry. It aligns all my criteria for a job that’s good for me. I’m able to travel, meet new people, help them with their projects, and do a lot of networking in the industry. It’s also very satisfying to know that my work has a positive impact on the quality of products. It’s a very rewarding job for me.
I’m happy with the way it happened, but I know that I would have different advice for someone starting out now. In my next post, I’ll give my suggestions for people getting their start in the industry. I hope it’ll help people get an advanced start.